Kevin Knudtson’s DNA Facility at the University of Iowa acquired a Pippin Prep almost a year ago, and in the time since, the automated size selection tool has become an integral part of the core lab’s microRNA pipeline. “Pippin is part of that workflow,” says Knudtson, director of the lab. “We’re not even going to consider a manual gel extraction for microRNAs.”
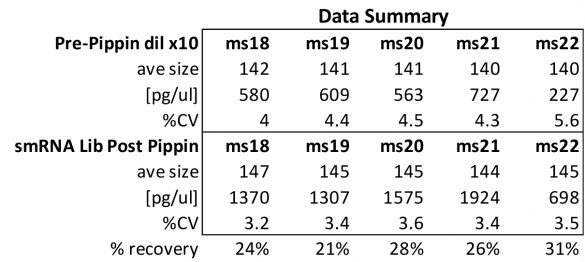
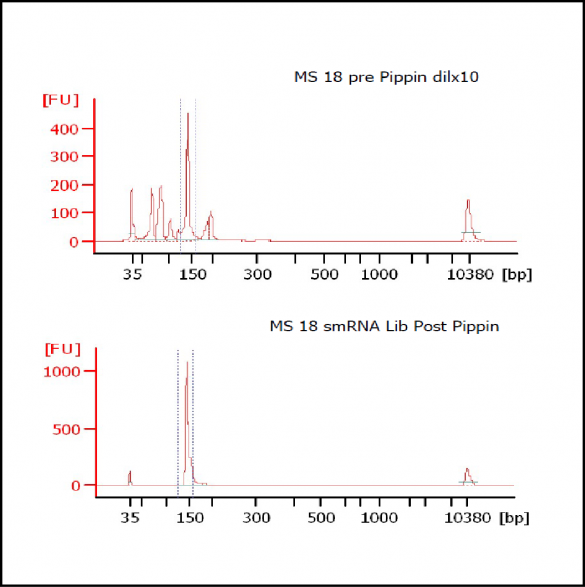
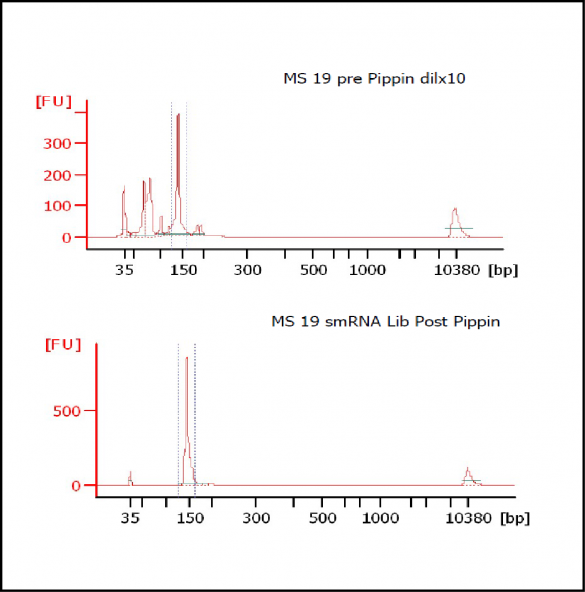
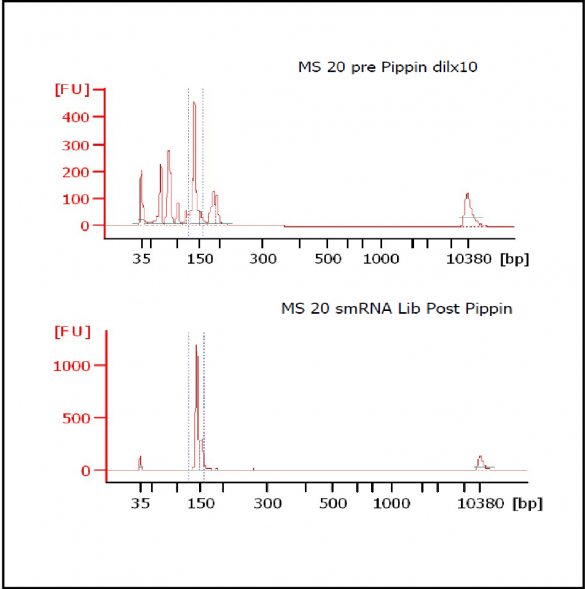
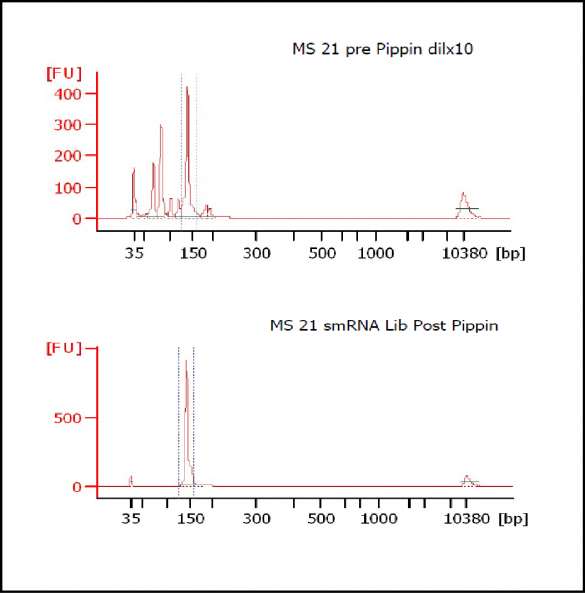
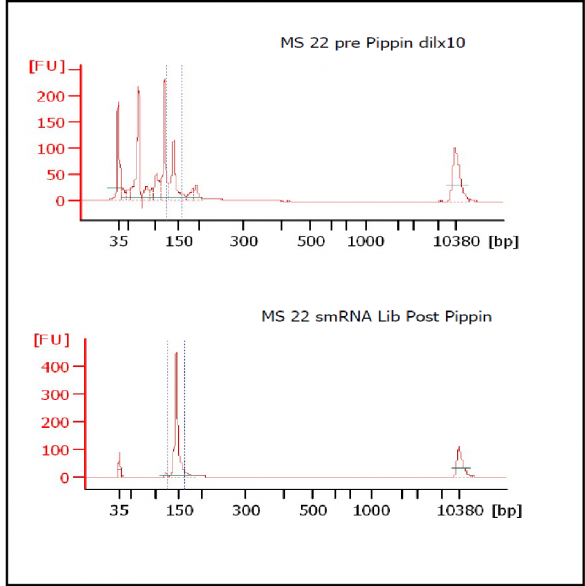
MiRNAs are especially tricky to extract from gels because the band of interest is near other bands containing unwanted products that adversely affect the quality of the sequencing run. “When you look at a Bioanalyzer trace, the band we’re after really is pretty small,” says Jennifer Bair, a member of Knudtson’s lab. “You see a lot of the other peaks much better.” The miRNA range of interest might be just 145 bases to 160 bases in size, but there will be quite a lot of adapter-dimers, primers, and other smaller content, as well as RNAs larger than the desired target. “We’re always amazed that the Pippin can isolate such a small fraction with such accuracy,” Bair adds. “It’s great.”
“For a microRNA process, there are a couple of bands that you do not want to collect,” Knudtson says. “Effectively excluding the unwanted or contaminating bands is essential to having good-quality data.”
The core facility brought in the Pippin Prep size selection instrument from Sage Science last year to replace manual gels, which are time-consuming and prone to inconsistency. “Cutting a slice out of a gel is a very subjective thing to do, and it can be technically challenging to repeat that same cut,” Knudtson says. “But when we run these out on the Pippin Prep, we’re getting exactly what we hope to receive. I can have different technicians do the same procedure and essentially get the same band or answer back when they’re processing samples.”
Knudtson’s team deploys automated sizing for more than the miRNA workflow. It has been useful for next-gen sequencing sample prep in the Iowa lab, which has a range of instruments: 454, HiSeq, MiSeq, and PGM. It takes a lot of samples to feed that pipeline, Knudtson notes. “Anything we can do to streamline the workflow is appreciated,” he says. “The Pippin Prep helps to shorten that process — it has sped up the turnaround time of samples.” Automated size selection also contributes to more efficient loading of flow cells, letting Knudtson get the most bang for his buck with sequencing runs.
The scientists have also begun to use the Pippin for ChIP-seq experiments, in which shearing chromatin to the correct size can be a real challenge, says Bair. While they are still evaluating how much size selection helps with this particular process, Bair says that generally the libraries can be made more efficiently without the larger fragments that sometimes get incorporated during the ChIP pulldown step.
Knudtson is pleased to have the days of manual gels behind him. From now on, he says, “if the protocol requires a gel extraction, we’re going to use the Pippin Prep to do it.”