Illumina Workflow: Pippin for ChIP-seq
As we continue our blog series on applications that are frequently used with Pippin size selection and Illumina sequencing, we move on to ChIP-seq. One of the most popular capabilities enabled by next-gen sequencing, ChIP-seq (or chromatin immunoprecipitation sequencing) is used to map protein binding sites or analyze protein-DNA interactions across entire genomes.
One of the earliest app notes for Pippin Prep came from Thomas Westerling at the Dana-Farber Cancer Institute. To learn more about the ChIP-seq library prep, check out the app note.
Pippin has also been used with large-scale ChIP projects, such as generating a complete picture of regulatory networks in Mycobacterium tuberculosis. This effort, tackled by James Galagan and his lab at Boston University, entailed methodically performing ChIP-seq on each transcription factor in the microbe to determine which other transcription factors and genomic regions were expressed as a result.
Chris Mawhinney, the scientist in Galagan’s lab who performed the work, told us that enlisting Pippin for these experiments saved time and eliminated the possibility of sample cross-contamination. “If you have too broad a range of sizes, the sequencer software has issues locating the clusters,” she said. “With Pippin Prep, you can get it down to a really nice, narrow size.” Proper sizing also removes adapter-dimers, which otherwise eat into sequencing capacity.
Mawhinney also told us that Pippin speeds up the sample prep routine. “The great thing about Pippin is that you can go right from size selection to PCR; there’s no middle cleanup step, so it’s very convenient,” she said.
We’ll continue our blog series in the coming weeks with posts on ddRADseq, Moleculo, and more. Check back soon!
Illumina Workflow: Pippin with Nextera
In this blog series, we’ve been looking at how Sage Science customers use their Pippin Prep and BluePippin instruments with their Illumina sequencers. Today we check out the Nextera workflow, which is designed for speedy NGS sample prep. It can yield even better results with a Pippin size selection step.
We visited Zach Herbert, associate director of the genomics core facility at Dana-Farber Cancer Institute, to learn more about how he built a Nextera+Pippin pipeline. He deploys the Nextera tagmentation protocol for small genomes and larger amplicon projects that come to the core lab. Herbert found that adding a size selection step with Pippin Prep afterward led to very tightly sized libraries. This method is a boon for reproducibility, MiSeq flow cell clustering, and data analysis.
Herbert also uses Pippin when he’s running samples together. Attempting to pool samples with a broad size range in equimolar amounts is very tricky — “but if all those libraries are the same size, then we’re much more likely to get an even distribution of that pool,” he told us.
Pippin and Nextera also work well together for mate-pair sequencing. Illumina recommends using the Pippin platform to get “more stringent” sizing than can be accomplished with AMPure alone. (You can find us under Size Selection in Chapter 3, beginning on page 40 of the guide.)
Check back soon for our next blog in this series. We’ll be looking at using Pippin in the Illumina sequencing pipeline for double-digest RAD-seq, ChIP-seq, and more.
1,000th Pippin Customer: Cave Scientist Extraordinaire
 At the University of Akron, geology and biology professor Hazel Barton shows her students the ropes. Literally. A veteran caver, Barton combined her passion for exploring some of the most remote locations in the world with her scientific interest in microbiology and became a widely acclaimed expert on the communities of extremophiles living in caves.
At the University of Akron, geology and biology professor Hazel Barton shows her students the ropes. Literally. A veteran caver, Barton combined her passion for exploring some of the most remote locations in the world with her scientific interest in microbiology and became a widely acclaimed expert on the communities of extremophiles living in caves.
Here in the hallways of Sage Science headquarters, Barton is famous for another reason: she is the 1,000th customer of our Pippin automated DNA size selection platform. As we celebrate this milestone, we wanted to share a quick profile of Barton’s science here on our blog.
Barton went on her first cave expedition on a class trip at age 14, and has been caving ever since. She never expected this interest to mix with her career, until her postdoc advisor and 16S sequencing pioneer Norman Pace pointed out that she could get to places on Earth that most people couldn’t, giving her a remarkable opportunity to study microbiology in unexplored realms. “Rather than my hobby being something that was a distraction from my science, all the years of experience I had in caves allowed me to go in and recognize things that were unusual and that could be biological,” she says. Today, her research sites include caves in Kentucky, New Mexico, Venezuela, Brazil, Belgium, and China.
When Barton first began her explorations with microbiology in mind, conventional wisdom suggested that the physical isolation and lack of sunlight characteristic of caves would mean they couldn’t support much life. Barton’s early research demonstrated that there was actually “a profound level of diversity,” she says. “I spent the rest of my career trying to figure out why caves are so diverse and what are the community interactions that support that diversity.” She has unearthed insights into relationships that allow these extremophiles to survive, from the microbes that work together to break down scarce nutrients to share an energy source to the “cheaters” that eat bacteria and even out population levels.
For Barton and the students who accompany her into the depths of these caves — a favorite sampling site is some 1,500 feet underground — extracting DNA is no simple task. “These microbes are ingrained in the rock,” she says. “It’s very hard to study them.” A gram of soil, for instance, might contain four to five orders of magnitude more bacteria than a gram of cave sediment. To make things even more challenging, less than 0.1% of those microbes can be cultured back in the lab.
That’s why Barton, whose team specializes in generating libraries from low-biomass samples, turned to the Pippin Prep. Her lab had been finding it difficult to get enough product for 454 sequencing, but scientists at a collaborating center in Kentucky “would send us these beautiful images of these gels” that they had generated from the same sample. “They could effectively get 4 nanograms of product and we would just lose it all when we would gel purify,” says Barton. When she asked why, she learned that the center’s success was due to Pippin.
She brought Pippin Prep into her own lab for testing and decided it was a no-brainer to purchase the instrument. “For the first time in a long time, we were able to clone products that we hadn’t been able to clone before because there just hadn’t been enough DNA,” Barton says. “The Pippin is so valuable to us because it’s all about very low biomass and getting a high percentage recovery.”
Many thanks to Barton and all the amazing Sage Science customers who helped us get to this point. Here’s to the next thousand!
Illumina Workflow: Pippin for RNA Studies
Illumina sequencers are far and away the most popular platform on the market. Since so many Sage customers use their Pippins with these instruments, we’re taking a look at some of the most common or interesting applications of automated DNA sizing with the HiSeq, MiSeq, and even the GA II or GA IIx workhorses. If you missed it, check out our recent blog about using Pippin for Illumina mate-pair sequencing. Our focus today is small RNAs.
Customers like Kevin Knudtson from the University of Iowa and Stuart Levine at MIT say that Pippin Prep is an integral part of the miRNA pipeline. Knudtson, who runs the university’s DNA Facility, says his team will not even “consider a manual gel extraction for microRNAs.” In their hands, Pippin is remarkably effective at isolating miRNAs, even with a lot of adapter-dimers, primers, and other small content in the neighborhood.
According to Levine, director of MIT’s BioMicro Center, “The high percentage gels on the Pippin allow us to cut out bands of the right size for microRNAs.” In a core facility, the alternative — manually cutting bands from a gel — is not economically feasible. His team also uses Pippin Prep for splice variant analysis with an RNA-seq workflow. “Some of the RNA-seq methodologies, when you’re doing de novo sequencing of transcriptomes and want to do assemblies, tend to perform better when the size distribution of the library inserts is very tight,” he says.
We also worked with New England Biolabs as they developed their NEBNext Multiplex Small RNA Kit, which functions best with Pippin sizing. A recent paper from scientists at the Mayo Clinic, Institute for Systems Biology, and University of Illinois demonstrated this workflow in a project examining the effects of vitamin D on microRNA regulation of gene expression in zebrafish. They enriched for miRNAs of interest with Pippin and the NEBNext kit. To learn more about how these products fit together, check out this app note.
Our blog series will continue with peeks into how scientists are using Pippin and Illumina sequencers together for ChIP-seq, ddRADseq, and more. Check back soon for more!
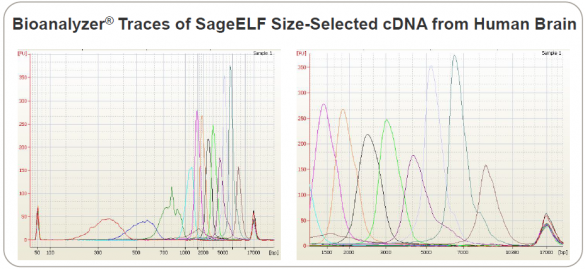
Poster on Full-Length Transcript Sequencing Includes SageELF
We are pleased to report the first customer poster featuring the newest addition to our product lineup, the SageELF whole-sample fractionation tool. “Single Molecule, Real-Time Sequencing of Full-length cDNA Transcripts Uncovers Novel Alternatively Spliced Isoforms” comes from scientists at Pacific Biosciences and the University of Washington.
The poster describes a human gene expression study in which the scientists were able to generate full-length isoform sequences, with some transcripts longer than 10 Kb. Isoform sequencing was conducted using the PacBio® RS II DNA Sequencing System. The cDNA sequences came from the MCF-7 human breast cancer cell line as well as from brain, heart, and liver cells.
The authors note: “Even in extensively profiled sample types, the method has been able to uncover large numbers of novel alternatively spliced isoforms and previously unannotated genes.” This ability to produce full-length transcript sequences offers a unique way to examine alternative splicing in eukaryotic organisms.
The team used SageELF, an automated sample prep device that generates 12 contiguous fractions from a single DNA or protein sample. It can be used in NGS workflows to build libraries with multiple insert sizes from the same sample, as well as to preserve precious samples. This Bioanalyzer trace of SageELF fractions is excerpted from the scientific poster: